Immunotherapy of Uterine Myoma
 What is uterine myoma and its symptoms
What is uterine myoma and its symptoms
Uterine myoma – benign tumor formed from muscular and connective tissues, which is one of the most common tumors found in women's reproductive organs.
Depending on the prevailing type of tissues- parenchyma or interstitial, this tumor was used to be called differently: myoma, fibroma, fibromyoma. However, since the myoma nodules are developed mainly from muscle cells, the most correct terminology is considered to be myoma (leiomyoma).
Uterine myoma is the most common pelvic tumor and they are diagnosed in upto15 to 20% of women in pubertal period [1].
Although myoma (uterine fibroids) is generally considered to be a slowly growing tumor, in 20-40% of women at the age of 35 and more have uterine fibroids of significant sizes with severe clinical symptoms [2]. Moreover, myoma can be relapsed in 7-28% of patients after surgical treatments and in certain cases it may even turn into malignant tumor.
The researchers have remarked that women who gave birth to at least two children have 2 times less risk of developing myoma than the childless women. Though, the scientists are not sure at this point whether the child delivery actually protects them from myoma, or if the myoma itself is the causative factor of infertility in those childless women.
For the growth of thus formed tumors, they need to be further supported by negative factors: abortions, long term use of inadequate contraceptive pills, chronic, sub acute and acute inflammations of uterus or its appendices, stresses, ultra-violet irradiation, cystic formation of ovary etc. For example, the women who had 10 abortions by the age of 30 have double the risk to develop uterine myoma at 40 years of age.
The growth of uterine myoma is featured as a benign, hormone sensitive diffuse or nodulous hyperplasia of myometrium and characterized by having multiple factors of pathogenesis, systemic changes, though the exact etiology of myoma is not known yet.
Uterine myoma is developed on the background of hyper estrogens, progesterone deficits and hyper gonadotropins. The majority of the researchers consider, that the growth of myoma depends on concentration of cytosolic receptors to the sexual hormones, and their interactions with the endogen or exogen hormones. In accordance to clinical observations, it can be admitted that both growth and regression of myoma are estrogen-dependant; the tumor size gets increased during pregnancy and is regressed after menopause [3]. The only moment that needs to clear is to find out whether it is the decrease in receptor numbers or estrogen, progesterone and androgen hormone quantities which lead to regression in myoma size (regarding androgen – there is an hypothesis that myoma is sensitive to androgen).
The chromosomal anomaly (12q13-15) is quite common in myomatous cells [4]. In fact, in 30-40% cases, the predisposition to uterine myoma is passed down from mothers to daughters on hereditary line. A form of myoma so called 'family type' is present where uterine myoma are seen in all the family line – in grandmother, mother, aunts and sisters.
Uterine myomas are often identified during routine gynecological examinations. In such examinations, the gynecologists may only assert the fact – presence of uterine myoma. In other cases the primary symptoms of myoma may appear as: hypo gastric pains, low backaches, bleedings, impairments of close up organs –example, tachyuresis (frequent urination).
We need special examinations to determine the number of nodules, sizes and their exact localizations. Mostly ultrasound examinations are enough for this, but in certain cases CT scans, MRI could be necessary.
Myoma may be located in the external, middle or inner layers of uterus (subserous, interstitial and submucous). Nodules can be located in the isthmus (5%) or in the uterine body (95%) [5]. The most 'unpleasant' ones are those, which are located in the inner layer.
Such types of myoma deform the uterus cavity, and thus cause severe bleedings during menses resulting into low hemoglobin levels (this is why during iron deficit anemia in women, they should have gynecological examinations).
The conventional treatment options
The uterine myoma by itself is not an indication for operative method of treatment. Mainly it depends upon the patients' overall health condition, severities of the clinical symptoms and the sizes of the tumor.
The major indications for the operative methods of treatment are severe pains, fast growth rate of the nodules, arising suspicions about the malignancy of myoma, inflammatory changes in the tumor nodules and dysfunction of closely lying organs (urinary bladder, intestines etc.), infertility (when all other reasons are already excluded). If an experienced gynecologist recommends you an operative method of treatments, you should go for it as early as possible.
It is necessary to watch the dynamic changes of myoma (ultrasound examination with vaginal probe), which in most cases gives sufficient information, and neither operations nor other therapies are required. If a woman has periodic pains, discomforts in the pelvic regions, any NSAIDs may help to relieve the symptoms. In case of having considerable clinical symptoms gestagen therapy (Norcolut, dufaston, premalut, medoxyprogesterone acetate); or using gonadotropin-releasing-hormone (Gn-RH) agonists (joladex). Tranexamic acid in tablets can be used during menorrhagia to decrease the heavy bleedings. Contraceptive pills can also be used for it.
The application of danazole, having androgenic properties, decreases the myoma sizes but on the other hand causes adverse effects: harsh voice, hirsutism etc. More effective ones are the hormones, which develops pseudomenapause, such as buserelin (nasal spray) and gozerelin (one injection per month). It is important to take into account, that the hormonal therapy brings only a temporary relief of the patients' condition, at the same time it causes many adverse effects. For example, long term hypoestrogenemia leads to high risk of osteoporosis [1, 6], where as after the withdrawn of hormones the growth of myoma speeds up (in 20% relapses occur with in 12 months, while in 50% in 5 years [4]. The application of hormone therapy just to shrink the nodule sizes is unjustified.
There is one more target of the hormone therapy – to decrease the blood loss volume and time of operative manipulations during myomectomy or hysterectomy.
The organ bearing laparoscopic myomectomy is done in the patients of age up to 40 years, having clear indications for surgical treatments. Particularly, it is expedient to resect out the macroscopic nodule of middle sizes (with diameter 2-5 cm), before they grow into too big sizes. While deciding to go for conservative myomectomy, it is also important to consider the morphological types of the tumors. For example, in proliferative types of myoma we can resect out several nodules, but many new nodules continue to grow further. This indicates that there are a high percentage of relapses (15-37%) after such conservative myomectomy. Moreover, the operations have common surgical risks including severe complications.
By now one more noninvasive method of treatment – transcatheter embolization of myomatosis nodules – as been elaborated, in which the nudules are cut off from blood supply and they regress out quickly with out affecting the uterus body as a whole. This method is still under clinical investigations and thus is not yet in wide use [7]. Various results of embolization in relation to the infertility rate and relapses of nodules are being analyzed.
After 40 years or in post menopause period, if there are clear indications for surgical treatments the patients should go for resection of the myomatous uterus (uterectomy), since even if the myomatous nodules do not grow much during the first 2 years of menopause, there is a high risk of changing them further into malignant tumors (adenocarcinoma, sarcoma).
Immunotherapy using vaccine RESAN
The progression of myomatous nodules or their growth can be controlled by checking the production of myogenic elements around the nodules, at the same time stimulating the apoptosis of myocites in the central regions of nodules. Regarding this, there are no conventional treatment options, which possess these clinical properties. It is the immune correcting therapy, which have these necessary clinical effects. The establishment of immune reactivity, immune correcting therapy, needs special approach to stimulate the immune system. A very new approach – an immunotherapy using tumor vaccine RESAN, which triggers specific T-cell immune response against uterine myoma has been elaborated [9, 10].
The components of immune system discern the particular surface antigens situated on the cell membranes. A very important role plays the proportion of cellular surface adhesion molecules – mainly E-cadherine [11, 12, 13, 14] and the tumor associated antigens CA125, CA19-9, CA15-3. Though, the mitotic activities of the uterine myoma are very low, the myomatous cells express oncoproteins of proliferation (Ki–67) and the oncoproteins which suppress apoptosis (bcl–2, bax).
In these respects, we recommend the application of tumor antigen imitators, included in the structure of a vaccine RESAN, with the purpose of correcting immune system for the treatment of fibromyomatous patients [10].
Patient's eligibility to RESAN immune therapy:
- Young ages (reproductive, pre menopause)
- Relatively small sizes of the myomatosis nodules (up to 10-12 weeks)
- Intermuscular localization of myomatosis nodules
- Relatively slow rate of myoma growth
- Absence of uterine cavity deformations (i.e. central growth and submucous localization)
To certain extent, we can draw analogy of indication for RESAN therapy to organ conserving surgical treatments at complete safety of vaccine application.
Next we represent a short reviewed protocol of our clinical assessment of patients suffering from different genital pathologies, who were treated with antitumor vaccine RESAN (immunotherapy).
We observed 35 women, among which 12 were suffering from genital endometriosis – adenomyosis, posterior isthmus form of endometriosis, external genital endometriosis; 13 – uterine myoma; 10 – combined form, endometriosis with uterine myoma; 2 – ovary cysts [8]. In all the patients we observe dishormonal hyperplasia of breast. In history, the patients were treated with different methods including hormonal therapy, immunomodulators, antioxidant therapy. Nevertheless, there were little clinical improvements from the applied therapy, i.e. some temporary (2-3 months) improvements in clinical symptoms (pain relief and some betterment in overall health conditions). As there were lack of necessary clinical effects from the previously applied therapy, we administered a course of immunotherapy using xenogenic vaccine which imitated the endometriosis and uterine myoma related antigens [10]. The vaccine was administered intracutaneously in one shot to all the patients.
The patients were examined before and after the vaccine treatments. The intensity of pains were measured in a "9 units" marking systems of Mac Laverty.
Pain in pelvic region, not associated with sexual intercourse or menses (3 units – severe, sharp, almost constant in which the patients were compelled to take analgesics; 2 units – moderate, tolerable, appreciable discomforts during the whole period of menses; 1 – mild, occasional discomforts or during pre menses period);
Algodysmenorrhea (3 units – severe, compels the patients to remain in bed for one or more days; 2 units – moderate, compels the patients to remain in bed for several hours in a day, occasionally loose working capacity, 1 unit – mild, with certain loss of working capacity);
Dyspareunia – pain during sexual intercourse (3 units –severe pains, have to avoid sexual intercourses; 2 units – moderate, have to interrupt sexual intercourse, 1 unit – tolerable pains);
Dysmenorrhea (3 units – blood smears seen 4-7 days pre menses; 2 units – blood smears seen 1-3 days pre menses; 1 unit – occasional appearance of blood smears pre menses);
Intensity of menses (3 units – copious menses, 2 units – moderate, 1 unit – scant menses).
Bimanual examinations, ultrasound examinations of pelvis and breast, mammography were conducted in all the vaccinated patients. The serum tumor markers (CEA, CA-125, CA19-9, CA-15-3), antigens of hepatitis B virus (HBV), antibody titers to Chlamydia trachomatis, Toxoplasma gondii, Mycobacterium tuberculosis and hepatitis C virus (HCV) were measured through immunoenzime analysis method before the vaccination and during the follow-ups in one month later [applied lab instruments and lab supplies- photometer STAT FAX 303 PLUS, auto-dosator BIOHIT and reagents from HOFFMANN-LA-ROCHE, HUMAN, BCM DIAGNOSTICS LLC, LABODIA-XEMA, VECTOR-BEST applying GLP system].
Before the treatments, the pain symptom was in average 9 units in 27 patients, who were suffering from genital endometriosis and in combined form (endometriosis with uterine myoma)
In 8 of the myoma patients the pain syndrome were not above 5 units. Dysmenorrhea was evaluated as 3 units in all patients with genital endometriosis and combined form of uterine myoma and endometriosis, where as in patients with uterine myoma alone (13 patients) – 1 unit.
Heterogenic consistencies of endometriosis, areas of high tenderness on uterine walls and increase in uterine body volume were observed during bimanual examinations and ultrasound examinations of pelvis. In the uterine isthmus, moderate swellings and pronounced pain symptoms were noted. During ultrasound examinations, in 12 patients with endometriosis genitalia were found adenomyosis of III-IV stages. In the patients with combined forms (endometriosis and myoma uterine), together with heterogenic mosaic structures, some interstitial, subserous and submucous nodes (in 5 cases) of 22-23 mm diameter were found. The uterine sizes were enlarged upto 9-12 week sizes in all cases.
The ultrasound examinations of breast in all the patients, diffuse (23 patients) and fibrocystic (12 patients) changes were located. The ultrasound results were correlated with that of mammography.
The tumor markers were elevated with in benign tumor limits, in all patients before the treatments. The average value of CA-125 in the group as a whole was 44.7±1.34 U/mL, CA-19-9 28.9 ±1.5 U/mL, CA-15-3 53.2 ±4.3 U/mL, CEA 1.29 ±0.38 ng/mL.
According to different infections found in the patients, they were classified into: Hepatitis B and C carrier – 27 (out of 35), Chlamydia trachomatis – 18, Toxoplasma gondii – 12, Mycobacterium tuberculosis – 6. It is important to note that the clinical states of the patients, antibody titers to the given infections indicated the chronic persistent states with out any signs of exacerbation of the infection. The presence of these infections influenced somehow in the overall health conditions of the patients in the post vaccination period.
During 7-10 days after vaccination, 30 patients admitted weakness, while patients with chlamydia trachomatis noted joint pains. Mild nausea, malaise were felt by patients with hepatitis carriers.
Carriers of tuberculosis and Toxoplasma gondii infections practically showed no clinical signs of exacerbation, except in 2 cases where we observed mild activation of chronic bronchitis within 1-1.5 months after the vaccination. Then, their health conditions were normalized, and no other adverse effects were recorded during next 3-4 weeks of observation. In the beginning, there was a tendency of growth in serum antibody titers to the given infections, while during next 1.5-2 months the titers returned to initial levels.
After one month, all the patients were followed up again. During bimanual examinations of the patients suffering from endomeriosis, the uterus sizes were shrank considerably and endometrium tissues became more homogenous with small areas of tenderness. In the cases of uterine myoma with nodules of different sizes, we observed considerable shrinkage in nodules and uterine body sizes. Where as in cases of endometriosis genitalia, the positive results were even more pronounced. During ultrasound examinations of genitalia, we observed a marked reduction in genital endometrioma and uterine sizes in patients suffering from endometriosis, where as in patients suffering from endometriosis in combination with uterine myoma, we noticed a decrease in interstitial, subserous and submucous node sizes (down by 16-26 mm). In all cases, the uterine body sizes were shrank to 5-11 week sizes (Fig.1).

Fig. 1. Sizes of uterusIn 7 patients were observed normalization of uterine body sizes, while in ultrasound examination there were a complete disappearance of myoma nodules. Similarly, in 2 patients we observed a complete disappearance of ovary cysts of sizes from 14.5 cm3 to 26.4 cm3 after the vaccination.
In ultrasound examinations of breast of patients with diffusing mastopathy (fibrocystic breast disease), already through one month after the vaccination no pathological changes in the breast tissues were admitted. In case of fibrocystic form of masthopathy the lesions of cystic formations were decreased in 1.5-3 mm sizes. The ultrasound results were correlated with that of mammography.
The pain symptom in 22 patients suffering from endometriosis genitalia as well as in combined forms (endometriosis and uterine myoma) was relieved from 9 down to 5-7 units (Fig. 2).

Fig. 2. The intensity of pain symptom
One month after the vaccination, the severity of dysmenorrhea was reduced to 1 unit in the patients with endometriosis as well as in those with combined forms (endometriosis and uterine myoma), where as in the patients (13) with uterine myoma the dysmenorrhea was corrected to 0 units (Fig. 3).
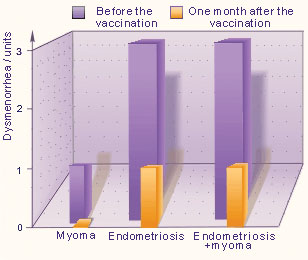
Fig. 3. Dysmenorrhea
We did not observe any clinical declines in direct correspond to vaccination in the patients. Patients rather admitted a decrease in edema, pain releases in the areas of estrogen-dependant organs, betterment in general health conditions and overall lifestyle.
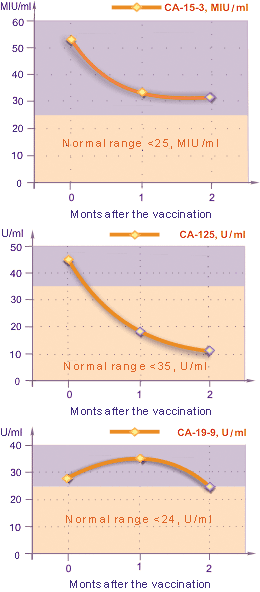 Fig. 4. Changes in tumor marker levels
Fig. 4. Changes in tumor marker levels
The changes in the tumor marker levels varied depending on the types of tumor markers (Fig. 4). The CA19-9 level after the vaccination, in average, went up by 23% (P < 0.05) from the initial value during the first month, but after two months it became 15% less than the initial value. The CA-125 level, in average, dropped steadily down to 25% (P < 0.05) from the initial value. The CA15-3 level fell down to 38% (P < 0.05) from the initial value, already in the first month after the vaccination. The average value of CA-15-3 in 2 months after the vaccination was 31.0 U/mL (P < 0,1%). In overall, these changes in tumor marker levels showed positive results of immunotherapy.
After evaluating closely the results of above clinical assessment, we came to conclusion that the application of vaccine RESAN considerably improved the general health condition and overall parameters of objective examinations in all the vaccinated patients who were under our observation.
We recommend the patients to examine for chlamidian infections, tuberculosis as well as for HBV and HCV carriers before the vaccination. In case of spotting high titers of antibodies against these infections, it is necessary to carry out a 'sanitarying treatment' of patients (using antibiotics, interferons, interleukines) before vaccination.
The immunotherapy of endometriosis and its combined form with uterine myoma by xenogenic vaccine RESAN results into regression or complete destruction of endometriomas, myomatous nudules and cystic formations of ovary.
References:
1. Gleicher, N. (1995). Immune dysfunction - a potential target for treatment in endometriosis. British Journal of Obstetrics and Gynaecology. 102 (Suppl.12), 4-7.
2. The Oxford Clinic – The Endometriosis Treatment Centre. Online.
3. IVF.com, Atlanta, USA. – Endometriosis Association, Education Support Research. Online.
4. Article: Uterine fibroids. Review Date: 04/09/01 Reviewed By: Catherine S. Bradley, M.D., Department of Obstetrics and Gynecology, University of Pennsylvania Medical Center, Philadelphia, PA.
5. Ballweg, ML. (1995). The Endometriosis Sourcebook. The Endometriosis Association. Contemporary Books.
6. Duczman, L. & Ballweg, ML. (1999). Endometriosis and Cancer: What is the connection? Endometriosis Association International Headquarters.
7. Senturk, LM. & Arici, A. (1999). Immunology of endometriosis. Journal of Reproductive Immunology. 43(1) 67-83.
8. Vinatier, D., Cosson, M. & Dufour, P. (2000). Is endometriosis an endometrial disease? European Journal of Obstetrics, Gynaecology and Reproductive Biology. 91(2) 113-25.
9. Paul Dmowski, MD, Director, Institute for the Study & Treatment of Endometriosis (ISTE), Oak Brook, IL.
10."Deep endometriosis conundrum: evidence in favor of a peritoneal origin," Fertil Steril 2000 May;73(5):1043-6 (ISSN: 0015-0282) by Vercellini P; Aimi G; Panazza S; Vicentini S; Pisacreta A; Crosignani PG.
11."Liver Health & Endometriosis," Julia Chang, M. Sc.
12. The Center for Endometriosis Care, Atlanta, GA. Online.
14. "Endometriosis 2000: a Report," by Dr. Mark Perloe. Online.
15. "Non-Invasive Diagnostic Detection of Endometriosis," NIH Grant # 1 RO1 CA96575-01, submitted by Rosalyn Blumenthal, Ph.D., Member/Director Tumor Biology, Garden State Cancer Center.
16. Professor Stephen Smith, Head of investigation of cellular, molecular and genetic factors which regulate angiogenesis and embryo implantation, University of Cambridge/Department of Pathology. On-line.
17. Deborah Metzger, Ph.D., Director, and Andrew Cook, M.D., Co-Director, Helena Women's Health Center. On-line.
18. "Immunopathology of Endometriosis," by The Reproductive Research Center at the Cleveland Clinic Foundation, Cleveland, OH. On-line.
19. Gleicher N. Immune dysfunction – a potential target for treatment in endometriosis. Br J Obstet Gynaecol 1995 Oct;102 Suppl:12:4-7.
20. Gleicher N, Pratt D. Abnormal (auto)immunity and endometriosis.
Int J Gynaecol Obstet 1993; 40 Suppl: S21-7.
21. Gleicher N. Endometriosis: a new approach is needed. Center for Human Reproduction, Chicago, IL. Hum Reprod 1992 Jul; 7(6):821-4
22. The vaccinotherapy of patients with mastopathy, endometriosis and mioma of uterus A. Ovsienko, H. Doronina , V. Yanchenko. Journal of Immunopathology: http://iaci.ru/journal/ http://iaci.ru/journal/issue2/full_text/sec6ar2.pdf
23. Novikov D.K., Novikiva V. I., Derkatch U.N., Novikov P.D. The basic immune corrections. Vitebsk, 1998, pg. 106.
24. Yantchenko V.V., Yantchenko A.V., Yantchenko L. K. Immitators of the tumor antigens. The patent committee RB, appl. N970547, 1997.
25. Immunology, Infertility and IVF, Sher Institute for Reproductive Medicine. On-line.
26. Endometriosis Protocol. On-line.
27. Endometriosis: clinical symptoms, diagnosis and treatments. R.A. Saidov. // Department of obstetrics and gynaecology MMA of I.M. Sachenov. On-line.
28. Gaetje R. Kuteian S., Herrmann G. Invasiveness of endometriotic cells in vivo. // Lancet, 1995, V346, P463-1464.
29. Gamallo C., Palacios J., Suarez A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. // Am. J. Pathol., 1993, V 42, P987-993.
30. Inoue M., Ogawa F., Miyata M. Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. // Am. J. Clin. Pathol., 1992, V98, P76-80.
31. Jimbo H., Hitomi Y., Yoshikawa F. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cyst. // Am. J. Pathol., 1997, V 50, P73-1178.
32. Sayunov M.A.Clinical symptoms, diagnosis and treatments of nodulous type of adenomyosis. // Obstetrics and gynaecology- 1987. N_3. – pages 34-36.
33. Lapauxov D.A. Clinical and diagnostic moments in combined forms of benign tumors of uterus in pre menopause periods: Auto abstracts for PhD candidates, 14.00.01. – M., 1992. pg. 33..
34. New findings on pathogenesis of internal endometriosis. // M.M. Daminrov, B.I. Bakuleva and others. // Obstetrics and gynaecology-1993. ¹ 5. – pages 28-32.
35. Ferenczy A. Pathophysiology of adenomyosis. // Hum. Reprod. Update. - 1998. - Vol. 4, ¹ 4. – pages 312-322.