Carcinogens
The carcinogens (chemical, physical, virus) act upon many people during their life-time, which give rise to constant changes in nucleotide - sequence of DNA of normal cells. As a result of these changes, forms a new type of cells - the cancer cells, which gain new properties:
1) Ability to undergo interminable mitosis.
2) Ability of metastasis.
3) Ability to powerful counter stand the immune system.

Fig. 1. Mutation
The possibility of formation of a tumor depends on 4 conditions:
1) Dose of carcinogen.
2) The affecting-time of the carcinogens.
3) Genetic predispositions.
4) Presence of stimulators (estrogen, cholic acids, sodium chloride, phenobarbital etc.) and inhibitors (vitamin A, E, C, aspirin etc.) of tumoral growth.
During the tumor transformation the functions of different genes may be hampered. The basic molecules, which identify own or foreign bodies in organisms, are so called the major histocompatibility complex (MHC) molecules, in case of human body they are denoted as HLA - human lymphocyte antigens, encoded in the 6-th chromosome. The HLA system has a broad polymorphism represented by the gene complexes - HLA-A, B, C, E, F, G, DR, DQ, DP, TAP1, TAP2, LMP-2, LMP-7 and others. A part of the own proteins synthesized by cells are immediately split out by the multicatalytic proteasome complex whose subunits are the products of the genes LMP-2 and LMP-7. The main function of these genes is to reduce the sizes of the peptides in conformity with linking sites of HLA molecules. Fig. 2 and Fig. 3.
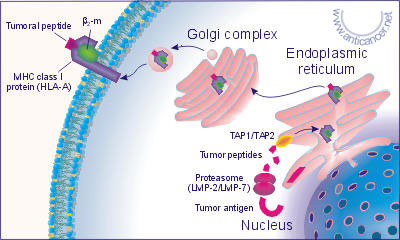 Fig. 2. The presentation of the peptide fragments of the tumor cell
Fig. 2. The presentation of the peptide fragments of the tumor cell
antigens in complex with HLA class I molecules
 Fig. 3. The nanomer peptide fragment of tumor antigen in complex
Fig. 3. The nanomer peptide fragment of tumor antigen in complex
with HLA class I molecule, A- side view, B- top view
Every organism has two sets of HLA genes, one inherited from father and the other one from mother. There are many varieties of HLA: HLA-A, B, C, E, F, G, DR, DQ, DP genes. They are called alleles and are denoted by numbers.
For example, the basic molecules of HLA class I human phenotype may have following varieties:
HLA-A3, 34; B14, 58; Cw6, 3. It is namely on these molecules depend which particular peptide fragments of tumor antigens in a complex with HLA the class I; molecules would be represented; on the surfaces of tumor cells and which one would not be. So in a person of HLA class I phenotype, the fragments of a Human cancer associated surface antigen (using the international single letter denotation for amino acids) would look like:
maitqfrlfkfctclatvfsflkrlicrsgrgrklsgdqitlpttvdyssvpkq tdveewtswdeaptsvkiegdgngnvatqqnsleqlepdyfkdmtptirktqki vikkreplnfgipdgstgfssrlaatqdlpfihqsselgdldtwqentnaweee edaawqaeevlrqqkladrekraaeqqrkkmekeaqrlmkkeqnkigvkls
And, on the surface of the tumor cells they will be represented quite differently, both quantitatively and qualitatively, in compare to that in the HLA-A1 phenotype. In a HLA-A3 phenotype person, most probably are represented by 35 different nanomer fragments of antigen while in the HLA-A1 phenotype only by 11 nanomer fragments, see Table 1 (the anchor amino acids are shown in bolted letters).
Table 1.
| HLA-A3 | HLA-A1 |
| Initial position, a nanomer peptide fragment | Initial position, a nanomer peptide fragment |
| 2 AITQFRLFK 7 RLFKFCTCL 14 CLATVFSFL 24 RLICRSGRG 25 LICRSGRGR 26 ICRSGRGRK 34 KLSGDQITL 39 QITLPTTVD 40 ITLPTTVDY 45 TVDYSSVPK 50 SVPKQTDVE 56 DVEEWTSWD 64 DEDAPTSVK 70 SVKIEGGNG 72 KIEGGNGNV 86 SLEQLEPDY 89 QLEPDYFKD 99 TPTIRKTQK 103 RKTQKIVIK 108 IVIKKREPL 118 FGIPDGSTG 119 GIPDGSTGF 130 RLAATQDLP 169 AEEVLRQQK 171 EVLRQQKLA 172 VLRQQKLAD 177 KLADREKRA 183 KRAAEQQRK 184 RAAEQQRKK 187 EQQRKKMEK 191 KKMEKEAQR 194 EKEAQRLMK 195 KEAQRLMKK 199 RLMKKEQNK 203 KEQNKIGVK |
16ATVFSFLKR 36 SGDQITLPT 40 ITLPTTVDY 54 QTDVEEWTS 86 SLEQLEPDY 89 QLEPDYFKD 112 KREPLNFGI 134 TQDLPFIHQ 143 SSELGDLDT 185 AAEQQRKKM 194 EKEAQRLMK |
Tumor Antigens
On the cell surfaces there are many antigens fulfilling different functions necessary for the vital life-activities of the cells.
A huge number of monoclonal antibodies (MCA) against these tumor antigens were obtained (some of the antigens has more than one name). The International Society for Oncodevelopment Biology and Medicine (ISOBM) regularly conducts conferences on the "Tissue Differentiation (TD) Works" in order to characterize MCA directed against the molecules, which could serve as the tumor markers.
Some antigens, which get bounded with human tumors, are present in fetal tissues, but are absent in same kind of tissues of adults. These antigens are called the oncofetal antigens. They play an extremely important role in tumor body growth. In fetal tissues, these are present as polypeptides whose synthesis is controlled by genes, and provide the cells an effective metabolism. After birth, sites of genes coding these polypeptides, lose their activities and further synthesis of fetal antigens is ceased for ever. But in tumor cells, these genes get repeatedly activated. In difference to those in fetal-tissues, these oncofetal antigens are glycoproteins. They are formed as a result of posttranslational glycosylation of fetal proteins.
Oncofetal antigens are present in spontaneous, virus- and chemical- induced tumors. They enable the tumor cells to have a high metabolism rate.
And so, the α- fetoprotein AFP (TD-2): an antigen with the molecular mass of 70 kDa. The AFP is a serum tumor marker for the hepatocellular carcinomas. The receptors to such AFP are located in many human cancers.
CEA (TD-8), the carcinoembryonic antigen, is a glycoprotein with the molecular mass of 200 kDa located on the cell membranes. The elevated level of CEA is observed in 30% of the patients with cancers of lungs, liver, pancreas, breast, colon, head, neck, urinary bladder, cervical uterus and prostates. Where as in those cancer patients already with metastatic growths, the elevated level of such serum antigen is observed in 60% of the cases.
The oncofetal antigens are considered as a group of antigens so called the cancer associated antigens, more over the same kind of cancer associated antigens were located in the embryonal and the normal tissues. The only thing is that in the normal tissues these antigens were in very minor quantities.
There are antigens, which are present only in the tumor cells. They are known as the tumor specific antigens. Among these tumor associated or tumor specific antigens, used to develop anticancer vaccines, certain ones can be distinguished- particularly those found on tumor cell membranes but are absent in the normal cells. These antigens are the ones, which are coded by genes such as BAGE, GAGE, MAGE. For example, MAGE family is located in the X-chromosome and consists of 15 genes.
The MAGE-1 antigen is identified in 36% of the patients with melanoma and in a considerable number in the histological preparations of breast cancers, non small cell carcinoma of lungs, carcinoma of head and neck.
The MAGE-3 antigen is identified in 65% of the patients with melanoma and 48% with carcinoma of head and neck. The two other families- GAGE and BAGE genes code for the synthesis of similar tumor specific antigens.
CA 125 (TD-1) antigen is an ovary cancer specific marker. This glycoprotein with a molecular mass of 22 kDa is expressed on the cell membranes of the cancer cells. During the I stage of ovary cancer the elevated level of CA 125 is observed in 50%, during II stage- in 90%, during III stage- in 92% , and during IV stage- in 94% of cases. Although CA 125 is considered to be the ovary cancer specific marker, the elevated level of this marker is observed in some other diseases as well, for example, in 15% of breast cancer, 30% of lung cancer, 31% of stomach cancer, 67% of cirrhosis and in 100% cases of cirrhosis with ascite.
PSA (TD-3) is a prostatic specific antigen with molecular mass of 33 kDa and thus is denoted as the prostate cancer specific marker. The antigen is located in the normal tissues of the prostate glands. The serum PSA level rises during malignancy. The blood serum PSA level is determined for the differential diagnostics and to trace the efficiency of treatments.
MUC-1 (TD-4)- a mucinoid antigen is a cancer associated mucin. Mucin is mainly located in the cytoplasm of the normal glandular cells of breasts and ovary. Nevertheless, MUC-1 is immensely expressed on the surface membranes of the cancer cells during breast cancer, ovary cancer, thyroid cancer and lung cancer. MUC-1 has the molecular mass of 300 - 450 kDa. A rise in the MUC-1 level also may be observed during some of the benign tumors and certain other diseases: benign tumors of mammary gland, ovary, endometriosis, hepatitis, hepatic cirrhosis, fibrosis of lungs. Pregnancy and lactation may lead to an elevation of the MUC-1 level as well. The standard test system for the determination of this antigen is called in different ways: BCM, CA15-3, CA153, CA27.29, CF15-3, MCA, M12, M20, M22 and others.
Cytokeratins (TD-5). In tumoral cells takes place a process so called an aberrant expression of keratin which can be identified through MCA. More than 30 different MCA against epitopes of the cytokeratins have been obtained.
CA-19-9 (TD-6). The Sialyl Lewis A antigen is a cancer associated carbohydrate antigen. The serum CA19-9 level rises in some of the patients suffering from lung cancer, prostate cancer. The Sialyl Lewis A antigens were discerned through immuno-histological method with the help of MCA NS19-9 in 75.4% cases of the primary hepatocelluar carcinoma and in 78.8% metastases of the regional lymphatic nodes. CA 195 is similar CA 19-9. It is found elevated in the blood serum of 50-70% of patients suffering from cancers of alimentary canal of different localizations.
DUPAN-2, the predecessor of CA19-9 is sialyllact-N-tetraose (LSTa, sialyl-Lewis(c)). The DUPAN-2 antigen, located on the surfaces of the cell membrane, is found in all types of carcinoma of lungs including the squamous cell carcinoma. In case of the adenocarcinoma, both in small and non small cell carcinomas, the antigens are located on cell surfaces as well as in the cytoplasm. DUPAN-2 is also found in the Lewis-negative blood serum of the patients suffering from pancreatic cancers.
The changes in the expressions of the antigens in the blood group Lewis Y (LeY) which appear during the malignant transformation is also considered to be a tumor marker. The LeY antigen is expressed on the cell surfaces and in the cytoplasm of the cancer cells during hepatocarcinoma.
One more antigen has been found in the cells of many tumors- the human chorionic gonadotropin (hCG) (TD-7).
The antigen found in bone cancers is called the BONE ALKALINE PHOSPHATASE (TD-9).
The antigens described above together with many other tumor associated antigens are used in the immunodiagnosis of the tumors and to develop new cancer vaccines.
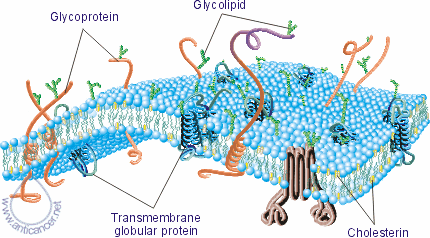
Fig. 4. The membrane of the normal cells

Fig. 5. The membrane of the cancer cells
The tumor cells secrete a factor which stimulates angiogenesis and thus possess a well developed capillary network. This in turn provide sufficient nutritious substances and growth factors needed for the fast growing cells. The tumor cells contain an enormous number of growth factor receptors encoded by oncogen fms. It allows to support an intensive body growth of a tumor.
Toxin-excreting glycoproteins and multiple drug resistance of tumor cells to chemopreparat
The lifespan of organism depends upon the capacity of its body cells to get rid of toxins- both exotoxins (entering from outsides) and endotoxins (formed during life processes) from the cells. For this, there is a whole group of ATP-binding cassette transporter proteins located in the plasma membranes. More the amount of toxins enters into the cells from out side or is formed in the cells; the more active becomes the transcription and translation processes of genes encoding these proteins. There are many medicines, which may alter the activeness of these genes.
There are 3 genes in the human chromosome locus 7q21.1: MDR1, MDR2 and MDR3, which encode the toxin-excreting glycoprotein. In the immunology very often the same protein has more than one nomenclature. Similarly, the products of the gene MDR1- the glycoprotien P, has the following names and symbols: ATP-BINDING CASSETTE, SUBFAMILY B, MEMBER 1; ABCB1; P-GLYCOPROTEIN 1; PGY1; MULTIDRUG RESISTANCE 1; MDR1; GP170; DOXORUBICIN RESISTANCE. The glycoprotien P (permeability) is located in the plasma membrane and its function is to excrete out metabolic products from cells. Its molecular mass is 170000 Da.
Due to huge numbers of these glycoprotiens in the tumor cells, they become so called multiple drug resistant. Thus, the tumor cells have capacity to excrete out quickly and effectively the chemo preparats used against them. The resistance of tumor cells to doxyrubin (adriamycin) is directly related to the activeness of the gene MDR1.
The products of the gene MDR3 — P-glycoprotein 3 — have the following names and symbols: ATP-BINDING CASSETTE, SUBFAMILY B, MEMBER 4; ABCB4; P-GLYCOPROTEIN 3; PGY3; MULTIDRUG RESISTANCE 3; MDR3.
In the same chromosomal locus 7q21.1 there is a gene encoding the protein SORCIN; SRI; MULTIDRUG-RESISTANCE COMPLEX, CLASS 4; MDR COMPLEX, CLASS 4 similar to glycoprotein P. The sorcin is a calcium-binding protein promoting the functions of calcium channels. The increasing activities of this protein make the tumor cells resistant to Vincristin.
In the chromosomal locus 3q27 there is located another gene encoding the fifth protein (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 5; ABCC5; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 5; MRP5; MOATC), which is resistant to multiple drugs. This protein is responsible for the tumor cells to be resistant to chemopreparats containing platinum.
The gene encoding the first protein (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 1; ABCC1; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 1; MRP1; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN; MRP) associated with the multiple drug resistance is located in the chromosomal locus 16p13.1. It promotes the transportation of leucotreins and protects from the heavymetals of oxyanions. The function of this protein is blocked by an inhibitor of 5-lypoxygenase.
The second multi-drug resistance-associated protein (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 2; ABCC2; MULTISPECIFIC ORGANIC ANION TRANSPORTER, CANALICULAR; CMOAT; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 2; MRP2) is encoded by the gene located in the chromosomal locus 10q24. It transports the organic anions and makes the tumor cells resistant to nucleotide-containing antitumor chemopreparats.
The gene encoding the third protein (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 3; ABCC3; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 3; MRP3; CANALICULAR MULTISPECIFIC ORGANIC ANION TRANSPORTER 2; CMOAT2) associated with the multiple drug resistance is located in the chromosomal locus 17q22. It promotes the transportation of organic anions and provides a resistance against the nucleoside-containing antitumor chemopreparats.
The gene encoding the fourth protein (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 4; ABCC4; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 4; MRP4; MULTISPECIFIC ORGANIC ANION TRANSPORTER B; MOATB) associated with the multiple drug resistance is located in the chromosomal locus 13q32 . It also promotes the transportation of organic anions and provides a resistance against the nucleotide-containing antitumor chemopreparats.
The gene encoding the fifth protein (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 5; ABCC5; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 5; MRP5; MULTISPECIFIC ORGANIC ANION TRANSPORTER C; MOATC) associated with the multiple drug resistance is located in the chromosomal locus 3q27. Similarly, it promotes the transportation of organic anions and provides a resistance against the platinum-containing antitumor chemopreparats.
There are two more genes located in the chromosomal locus 16q12.1; which encode the eighth and ninth proteins (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 11; ABCC11; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 8; MRP8), (ATP-BINDING CASSETTE, SUBFAMILY C, MEMBER 12; ABCC12; MULTIDRUG RESISTANCE-ASSOCIATED PROTEIN 9; MRP9) and are associated with multiple drug resistance.
In the chromosomal locus 16p13.1-p11.2; there is a gene, that encodes a protein (MAJOR VAULT PROTEIN, RAT, HOMOLOG OF; MVP; LUNG RESISTANCE-RELATED PROTEIN; LRP) which is also associated with multiple drug resistance to antitumor chemopreparats.
Considering these genes and their corresponding proteins making the tumor cells resistant to chemopreparats, it is quite clear why the century long studies and practice of chemopreparats against the tumors could not achieve satisfactory results. To envoy rather safer chemopreparats, it is necessary to carry out diagnosis at gene level and choose the right (non-resistant) chemopreparts for the patients. After a complete course of the first chemo preparat, it is always better to change the drug for the second time, since the initially used chemo preparat causes the tumor cells to produce toxin-excreting proteins 10 or more times. The second chemopreparat should also be chosen only after the diagnosis at gene level; in order to choose the preparat which would be the least vulnerable to excretion by the cancer cells that are left in the patient. Otherwise the chemopreparat would only weaken the immune system of the patient and cause high toxic effect to different organs and systems of the patient, practically having no effect on the tumor cell growth, and consequently increases the morbidity rather than to help the patient
T-Lymphocytes
The main agents of antitumoral protection in an organism are the cytotoxic T-lymphocytes or T-killers. On the surface of tumor cells the antigens are represented not only in the form of a single molecule, as shown in Fig. 5, but also in the form of fragments in complex with HLA molecules, Table 1, Fig. 2 and Fig. 3. The identification of a tumor cells by T-killers depends on the various antigens present in the tumor cells. For T-lymphocytes the sequence of amino acids of a polypeptide is important rather than how it is packed in the space. In order to get good response from T-lymphocytes to antigens, the antigens should be represented by an antigen presenting cell (APC).
B-lymphocytes, macrophages and dendrite cells are refered to these antigen presenting cells (APC) which are specialized in processing the foreign and own defect materials. The protein- antigens are swallowed by APC. Inside the cell these proteins are split into peptide fragments of length upto 10-20 amino acids. These peptide fragments shift toward the plasmatic membrane of APC and together with molecules of HLA form receptors, recognizable to T-lymphocytes.
For this purpose in T-lymphocytes present their own T-cell receptor (TCR). The TCR consists of peptide complex- a, b, e, g, d, z, h each of which are encoded by separate genes. The conjunction of TCR with the tumor peptide represented by the HLA molecule is usually not enough to fulfill the complete action of T-killer. Binding of one more molecule of T-killer i.e. CD8-co-receptor with a HLA molecule of target cell is necessary. TCR of T-killers and T-helpers are encoded by identical genes, but co-receptor for T-helpers is represented by different protein- CD4. The co-receptor- CD4 binds with HLA class II molecule where as the co-receptor- CD8 with HLA class I molecules. If a macrophage with HLA of the class I represents antigen to an immature T-cell, forms clones of T-killer cells. On the other hand, if macrophage with HLA class II molecules represents the antigen, then forms clones of T-helpers. In the human population there are many types of HLA proteins but in each individual present only two types of genes- for HLA molecules of I and II types.
The ability of T-killers to respond to tumor cells also depends on molecules MHC of the tumor cells. The molecules MHC of the class I are encoded by the two genes in tumor cells of mouses: H-2K and H-2D where as in the human tumor cells they are encoded by the following genes : HLA-A, HLA-B, HLA-C. The human HLA-A gene coincides with the H-2K gene of mouse. The CD8 co-receptor of T-killer effectively binds with a molecule H-2K but does not bind with the molecule H-2D.
The more the ratio of molecules H-2K:H-2D on plasmatic membrane, more the tumor cells become distinct for the T-killers. On the other hand, more the expression of a gene H-2D takes place the more often tumor cells escape from the immune attack by the T-killers in blood stream and accordingly more often metastases are formed.
The rate of expression of genes H-2K and H-2D is variable. The α- and β interferons when injected together induce the expression of H-2D gene, while the gamma interferon increases the expression of H-2K gene. Thus, expression of H-2K and H-2D genes can be regulated and, accordingly, increase the immune response of T-killers against tumors. Besides, the gamma interferon suppresses an angiogenesis.
T-killer, coming in contact with a tumor cell through their receptors, forms a close bond using Mg++ ions, and excretes out protein perforins. Perforins lay out on plasmatic membrane of tumor cell which in the presence of Ca++ gets polymerized forming channels through which enters exceeded amount of water into the cell and finally the tumor cell bursts out. Each T-killer destroys only a limit number of tumor cells after which the deposition of energy and perforins in T-killer cells get exhausted and they die out on their own.
NK-Cells
An important element in the anticancer system is so called the natural killer cells (NK-cells). The average number of NK-cells in a healthy person should not be less than 170 per ml blood serum. These cells take active parts in the antiviral and anticancer system of our body. The unique feature of NK cells is that they can destroy the cells in which there are low HLA class I molecule expressions.
The natural killer cells have different molecules, on which depend their cytitoxic activities. One of such molecules is CD16 (low-affinity receptor for immunoglobin G- IgG). The NK-cells can get attached to the cancer cells through antibodies- IgG (if they are present on the cancer cell surfaces) in the presence of CD16 molecules. The other important group of molecules present on the NK-cell surfaces are CD158 molecules. They are also called the immunoglobulin-like receptors of killer cells ( KIR) or the inhibiting receptors of the killer cells (KIR). The whole set of these KIR molecules are encoded by 12 genes. The different KIR molecules interact with different HLA class I molecules. In other words, the KIR molecules of NK-cells play a role of receptor for the HLA clas I molecules of our normal body cells (Table 2)
The inhibiting function in the NK-cells are also fulfilled by CD94/NKG2 molecules, which are rather specific for HLA-E molecules. In difference to KIR, the CD94/NKG2 molecules are related to lectin-like molecules. In general, all the nucleated cells of our organism contain the HLA class I molecules. The expression of these HLA molecules in the cells of our organisms can be changed during tumoral transformations or viral invasions in them. When there are low expressions of HLA molecules, the inhibiting function of KIR molecules in NK-cells remain deactivated and the abnormal cells get destroyed by the natural killers. In this way, the NK-cells protect our organism from the cancer cells with low HLA class I molecule expressions.
After the close contact of NK-cells with cancer cells, the NK-cell secrete out protein molecules so called perforins which lay out on the cancer cell surfaces to form pores. Then the NK-cells stand out from the cancer cells while through thus formed pores start to enter the inter-cellular fluid into the cancer cell. The cancer cell slowly swells out and at the end bursts out. On the NK-cell surfaces there are many stimulating molecules or activators. They are the receptors for IFN-g, IL-2, IL-12, IL-15, IL-18 molecues.On the surface of all the NK-cells present so called the FAS-ligand molecules (CD178), which can trigger a cell death program in the targeted cells. The activation of NK-cells by interleukin 2, interleukin 12 elicit an intensive expression of CD178 in NK-cells. In fact the interaction between CD16 and IgG also lead to the same results. In this way, the destruction of the cancer cells can also be elicited through the interaction of CD178 with the receptors of apotosis on NK-cells. This is one of the vital reasons why in people with low numbers and/or with low functional activeness of NK-cells develop oncological diseases considerably more frequently.
LAK-Cells
The activated lymphocytes called as the LAK- cells (lymphokine activated killer) can also destroy the tumor cells. The LAK-cells are formed from so called the "zeroth" lymphocyte population. Similar to the NK-cells, they destroy tumor cells in the first encounter, without preliminary sensitization by particular antigen. The antitumoral activity of LAK-cells increases if it is injected together with interleukin-2. Note: Big doses of interleukin-2 (IL-2) causes the toxic effect, resulting to expressed oedema of organs and tissues.
Macrophages
Tumor cells synthesize a factor which inhibits the migration of macrophages (MIF), which is a very necessary substance for tumoral body growth. MIF fulfils simultaneously two important functions in tumoral body growth. Under the action of MIF, the macrophages happened to be near the tumor, lose their mobility but their capacity to synthesize bioactive substances is retained. In this way, MIF deprives the macrophages to pass the information to other immune competent cells about the detection of tumor and allows the tumor to use macrophage as a factory which produces a huge amount of plasminogen-activators. With the help of the plasminogen activator, synthesized by macrophages, the tumor-cells penetrate into the blood vessels and spread out in the organism.
The macrophages when activated by T-lymphocytes transfer arginine into nitric oxide. This toxic substance kills tumor cells. It occurs as follows: the activated lymphocyte excrete an γ-interferon. It submits a signal whose target is the nucleus of macrophage. This signal stimulates the production of nitric oxide syntheses transferring arginine into nitric oxide. Nitric oxide in turn destroys tumor-cells, reducing the energy formation in Krebs cycle and during the transportation of electrons in mitochondrions. The nitric oxide reduces the synthesis of DNA. In this way, without arginine and the synthesis of nitric oxide macrophage can not fulfill its protective function. Methyl derivatives of an arginine, on one hand, blocks the formation of nitrates in macrophages on the other hand, restrains the ability of macrophages to destroy tumor cells.
B-Lymphocytes
During the interaction of tumoral antigen and B-lymphocyte, a selection of clone B-lymphocytes takes place, whose receptors correspond to the given antigen. They co-ordinate with tumoral antigens and get activated. In activated B-lymphocytes, processed antigens are located on plasmatic membrane together with MHC class II molecules. Mature T-helper cell, which has under gone specific activation by macrophage with MHC class II molecule, contacts with activated B-lymphocyte. It helps T-helper to gain the ability to excrete interleukin-2 under whose influences the B-cell undergoes mitosis and differentiation converting into a plasma cell. A mature plasma cell secretes antigen-specific immunoglobulins (antibody). This tumor-specific antibody contacts with specific antigens of a tumor. But the tumor cell has an interesting feature- it can lose the surface antigens (like a lizard loosing its tail when it is caught by it). The total complement level of human being is insufficient to develop an antibody dependant lysis of the tumor cell. The complex antigen-antibody leaves the tumor cell earlier than the activation and polymerization of complement take place. Generated antitumoral antibodies and the circulating immune complexes worsen the state of diseases. They block out antigens of tumor cells from receptors of T-killers, protecting tumor cells from cytolytic attack.
Carbohydrates
In the structure of carbohydrates, as in the nucleic acids and proteins, important biological informations are stored. In fact, the carbohydrates have the highest capacity to carry informations as they have the greatest potential to form most variable structures. For example, from two identical monosaccharides can be formed eleven various disaccharides whereas two amino acids can form only one dipeptide. The nomenclature of different carbohydrates is based on variation of sugar subunits; differences of bonds between them and the presence or absence of branching sites. The set of carbohydrates of cancer cells vastly differs from that of normal cells, and thus can be used as a marker. The carbohydrates located on the surface of tumor cells help to form metastasis. Having penetrated in blood stream, the tumor cell circulates in the blood system. The tumor cell can abandon the blood vessels only through the capillary wall of veins. In capillary of vein the tumor cells come in contact with E-selectins (special surface molecules of adhesion which inter react with surface carbohydrates of other cells) of endothelial cells and quit the bloodstream through the space between endothelium cells. In such a way the tumor cell fall into other tissues and organs forming metastases. The tumor cell needs 8-24 hours to abandon bloodstream completely. Only 1out of 10000 cells, which have been moved away from the primary tumor, survives to give a new colony.
The tumor cells, on their surface, have so called the cell adhesion molecules (CAM). These molecules, during the formation of metastasis, are attached to CAM of those organs and tissues, which are most similar to them. The more advanced the capillary network in an organ the more often metastases develop there. It explains the reasons why the most typical and common metastases of certain tumors occur in particular organs and tissues. CAM and the immunoglobulins have a structural homology. The molecules of immunoglobulins have been developed from CAM during the process of evolution.
Lectins (a type of protein capable to form quick, selective and reversible bonds with carbohydrates) have particular value in tumoral body growth. They, getting fixed with surface carbohydrates of tumor cells, become a factor, which protects tumor from the immune system. If the cells of melanoma are processed with chemical compositions containing lactose, their capacity to give rise to metastasis decreases almost twice.
The secondary tumors metastasize easier, as their vascular network formed as a result of a self-induced angiogenesis is more permeable. A close study of the mechanisms of interaction between tumor cells and immune system allows develop a new, effective drugs for the prevention and treatments of oncological diseases.
Interleukins
Today genes are well known with whose help the amino acid sequences of more than twenty interleukins (IL-1...IL-22) have been determined which play an important role in the formation of the antitumoral protections in the human body. During every tumoral growth there is a certain degree of intervention in the interleukin-system which is manifested as a disbalance in the production and regulation of these biologically active substances, changing the expressing mechanism of their respective receptors.
The interleukins are produced by different cells of an organism. They play an important role in interaction between the cells of all the organs and systems. In many case they act as the factors of autocrine regulation.
Fig. 6. Production of cytokines
Interleukin-1
Interleukin-1 (IL-1) takes part practically in all the phases of an immune response. They activate APC and CD4 lymphocytes, affect in the differentiations of the T-, B- lymphocytes and other immunocompetent cells. IL-1 activates the cytotoxic T- lymphocytes and NK- cells, takes part in the regulation of productions of IL-2, IL-4, IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF) and other cytokines. The active inhibitors of IL-1 produсtion are IL-4, IL-10, IL-12 and the tumor necrosis factor -alpha (TNF-a).
Depending on the certain types, IL-1 plays different roles in the tumor growth process. There are two types of this interleukin: IL-1a and IL-1b. They differ from each other in their bioactivities. IL-1a for example, strengthens the weakening antitumoral immunity, slows down tumor growth and lowers its metastatic potential, strengthens the resistibility against the bacterial infections where as they do not affect in the regeneration process of tissues. IL-1a easily gets inactivated in high body temperatures. The prostaglandin E-2 (PGE-2) decreases the antitumoral activity of IL-1a.
IL-1b, on the other hand, reinforces the regeneration of tissue and stimulates the metastatic processes in the oncological patients. It takes place because IL-1b activates the production of prostaglandin E-2 (PGE-2), increases the expression of mannose-receptors in the endothelial cells and the production of the tumoral growth factor. An increase in the level of tumor markers by 2-10 times was observed with in a month after the introduction of IL-1b in the oncological patients with out metastases; where as in the patients with metastases, there was an increase in the level of tumor markers by 20-40 times.
Many attempts have been made to neutralize IL-1b in oncopathology. For these purposes they use: monoclonal antibodies or soluble recombinant IL-1b receptors (sIL-1bR), blockage of IL-1b receptors by the recombinant IL-1b antagonists. But due to the short half-lives of the IL-1b antagonists in vivo and sIL-1bR, big doses of these proteins are required. Research works are being carried out towards the development of sIL-1bR-IgG of integrated protein, which circulates in blood for long time and provides better therapeutic effects. The introduction of the antagonists of a receptor IL-1b (IL-1bRa) inhibits the development of metastases by 73-87%.
Interleukin-2
Interleukin-2 (IL-2) possesses a high capacity to induce the activation of practically all the clones of cytotoxic cells. It was the first interleukin in which such capacity was located and was the first interleukin used by Stiven A. Rosenberg and his colleagues in the immunotherapy of cancers.
IL-2 increases the cytotoxic functions of T-killers and NK-cells, promotes the production of perforins and IFN-g by these cells, activates the monocytes and macrophages which help to synthesize and secrete TNF-a, IL-1b, IL-6, IL-8, granulocyte- colony stimulating factor (G-CSF) and GM-CSF.
The administration of IL-2 enables to speed-up the proliferation of T- and B- lymphocytes, build-up the immune response against T-dependent antigen, restore the functional reserves of the macrophages.
A positive effect was observed when a low doses of IL-2 were used in the treatment of the non Hodgkin's lymphoma. For this a long-term infusion therapy using recombinant IL-2 in the combination with monoclonal antibodies against CD19 is applied. IL-2 can give rise to a long-term remission of an acute myeloblastic leukaemia (AML). A complete remission can be gained by using IL-2 in the AML patients with a low level of residual blast cells in the bone marrow. Nowadays, in a number of clinics, IL-2 infusion therapy is applied as a supporting therapy in the secondary remission period of an acute myelocyte leukaemia. IL-2 also gives good results in the treatments of malignant melanoma, renal carcinoma and vascular endothelial sarcoma during their pre- and post-operational periods. The intranidus use of IL-2 in case of hemangioendothelioma often results into a complete disappearance of the tumor.
It is necessary to consider that during the use of high doses of IL-2 may cause a serious haemolytic disorders giving rise to anemia, neutropenia, trombocytopenia and lymphocytosis. Anemia develops due to the decrease in numbers of the colony of early precursors of the erythropoiesis in bone marrow caused by IL-2. One can not exclude the fact that the effects of IL-2 could be mediated through IFN-g, whose synthesis is induced by IL-2, as the IFN-g antibody interrupts the descension of erythroidal colonies. Similar influences of IL-2, intermediated by IFN-g, is also marked out in case of neutrophils.
Lymphocytosis is developed by a direct mitogenic effect of IL-2 on the cells from lymphoidal series.
In case of different malignant neoplasms, it may have a consideration in decreasing the IL-2 production by lymphocytes in the peripheral blood, which often correlates with the decrease in the killer cell activities. But, the authentic decrease in the IL-2 production is identified only in the late stages- III and IV stages when no apparent changes in the early stages of the process are notified.
The active stage of IL-2 is also dependent on the functional states of receptors IL-2 (IL-2R). So, in case of glyoblastoma we notice a specific defect in the IL-2 production and decrease in the IL-2R expression in the cytotoxic cells. The mutation of the IL-2 receptors is marked out in the lymphocytes of the patients with stomach carcinoma, colon cancers. During a simultaneous study of the levels of IL-2 production and IL-2R expression in the T-lymphocytes, in response to IL-2, in glyoblastoma patients- it was determined that decrease in the IL-2R expression is due to the low level of thyrosinum - phosphorylation in T-lymphocytes (in other brain tumors it was not noticed).
Considerable difficulties are met during realization of antitumoral activities and production of IL-2 due to the formation of the high concentrated soluble forms of IL-2 (sIL-2R) receptors. The physiological concentrations of sIL-2R in the healthy people regulate the interactions in cytokine network. Where as in cancer patients the considerable increase of sIL-2R results to a formation of immunsuppression. A high level of sIL-2R is found in the blood serum and ascitic fluid of ovary cancer patients. Similar rise in the sIL-2R level in blood serum is found in the cancer patients with melanoma, lung cancers, cancers of intestine, kidney and urinary bladder, which directly correlate with the progression and formation of metastases from the primary tumor. The high level of sIL-2R in the malignant neoplasms indicates a poor prognosis and high aggressiveness of disease.
It has been approved that the inhibition of IL-2 is associated with the accumulation of the immunsuppressive substances mainly- the prostaglandins, immune complexes and the metabolic products of cancer cells developing an immunosuppression state. The gangliosides excreted by the human melanoma, suppresses the production of IL-2 and render a direct destructive impact on its molecules.
Interleukin-3
Interleukin-3 (IL-3) is a poly potent activator of the hemopoietic cells. The exact role of IL-3 in the cancer growth had been studied insufficiently. Its involvement in the antitumoral defense may be through a stimulation of NK-cells and acts as a synergist with IL-4 during the induction of CD4+ lymphocyte activation process. IL-3 increases the tumoral cytotoxisity of the T-lumphocytes.
Interleukin-4
Interleukin-4 (IL-4) takes part in differentiation of T- helpers: Th-0 in Th-1 and Th-2. The B- lymphocyte synthesizes IgE under the influence of IL-4. IL-4 controls the production of TNF-a, IL-1b, IL-5, IL-6, IL-8; promotes the differentiation in cytotoxic T-cells; activates the macrophages and intensifies their cytotoxic potential; induces the proliferation of NK-cells and in certain conditions may take part in the generation of LAK-cells. The main producers of IL-4 are CD4+ and CD8+ lymphocytes, B-lymphocytes and macrophages.
In moderate doses IL-4 may be a synergist with IL-2 in inducing the LAK-cells, obtaining from the peripheral blood or from the lymphocytes of the tumor infiltrates. IL-4 in small as well as in high doses inhibits the IL-2 production by lymphocytes and IL-2 induced cytotoxicity of the LAK-cells, slows down the expression of IL-2 receptors. IL-4 inhibits in vitro the growth of the malignant cells in the acute lympholeukemia patients with Ph+ (Philadelphia) chromosome, where as in case of acute leukemia with Ph- the IL-4 does not affect. IL- 4 suppresses the growth of malignant cells in the patients with chronic myelomonocytic leukemia, possesses a considerable anticancer effect in the chronic myelogenous leukemia (CML) and in acute myeloblastic leukemia (AML), but does not effect in chronic lympholeukemia (CLL) with Ph+ during the blastic crises.
Interleukin-5
Interleukin-5 (IL-5) chiefly regulates the proliferation and differentiation processes of the eosinophils and basophils. The B- lymphocytes synthesizes IgA under the influence of IL-5. IL-5 plays an important role in allergic inflammations.
The IL-5 antitumoral activation is associated with it's capacity to take part in the apoptosis which is demonstrated in the experiment with the erythroleukemic TF-1 cells sensitive to IL-5 as well as with the capacity to induce the eosinophil activation. This in turn damages the tumor cells by excreting cation and basic huge proteins.
Interleukin-6
Interleukin-6 (IL-6) regulates the differentiation of B - lymphocytes and increases the antibody production, induces cytotoxicity of cells independent from the expressions of MHC antigens including their response to IL-2 and IFN-g. Alongside with its expressed pro inflammatory activities, it modulates the antitumoral activities of macrophages. IL-6 takes part in generation of LAK-cells and protects neutrophils from apoptosis increasing their cytotoxic potential in connection to the tumoral cells. IL-6 speeds up the synthesis of C - reactive protein (CRP). Having a five- dimensional form of C - reactive protein, after binding with phospholipids of a tumor cell, it activates the subcomponents C1qof the complement system, switching on the process very similar to the classical way of activation of complements which forms a membrane-attacking complex and in certain cases leads to a tumor cell lysis.
The inhibition of the tumor growth by IL-6 may be also associated with it's ability to induce a secretion of IL-1b antagonist receptors. IL-6 can induce regression of tumors only in the early stages of weak immunogenic tumor growth, where as it does not render such effects in the immunogenic tumor growth located in the late stages of their development. In most of the cases, tumoral progression is accompanied by an increase in the level of IL-1b, IL-6 and acute phase protein, for example in the tumors located in: head, neck, larynxes, stomach, liver, pancreas, intestine, kidneys and ovary. The progression of tumor growth is associated with the increase in the antibody productions under the influence of IL-6. These antitumoral antibodies block out the tumor cell antigens and the T-killer cell receptors, defending the tumor cells from destruction.
The antibodies to IL-6 slow down the tumor growth. Besides the antitumoral effect, the impact of antibodies on IL-6 reduces a CRP level and normalizes the neutropenia and thrombocytopenia in the patients with myelomonocytic leukemia. But, if IL-6 level in the blood serum of the cancer patients is very high then the appropriate effects from the applied doses of IL-6 antibodies can not be achieved.
IL-6 together with IL-1b takes part in pathogenesis of anorexia, cachexia and anemia in the oncological patients. Intensification of C-RP synthesis under the IL-6 action promotes atherosclerosis in the patients with the given pathology.
By blocking the genes or introducing the antibodies against IL-6, these clinical complications- anorexia, cachexia and anemia reduce to mild forms.
Interleukin-7
Interleukin-7 (IL-7) is known as the growth factor of the immature B- and T- lymphocytes and mature T- lymphocytes. IL-7 generates the tumor-specific T- killers of various localization, takes part in the generation process of LAK-cells, acting as a synergist with IL-2. IL-7 requires longer cultivation than IL-2 for the LAK-cells induction but the LAK-cells induced by IL-7 is more effective in terms of cytotoxicity and retains this property for a longer period which causes tumor cell lysis of a broad spectrum. IL-7 regulates the expression of IL-2 gene in the activated T-lymphocytes, therefore a drop in the production of this interleukin may have a negative impact on the IL-2 production as well. IL-7 can induce apoptosis of tumor cells, causes differentiation of cells from a subgroup of acute myeloblastic leukemia.
Interleukin- 8
Interleukin- 8 (IL-8) is produced by many types of cells and possesses a high anti-inflammative properties. The basic bioactivity of IL-8 is the induction of chemotaxis of neutrophils, eosinophils, basophils and other cells from immune system. IL-8 re-enforces angiogenesis in vivo and in vitro. The role of IL-8 in tumor growth is not investigated sufficiently yet.
Interleukin- 9
Interleukin- 9 (IL-9) stimulates the excretion of IL-2, IL-4, IL-6, IL-11, IFN-g. IL-9 takes part in a stimulation of cytotoxicity of T- killers and NK-cells, induces apoptosis. The role of IL-9 in tumor growth is investigated not sufficiently.
Interleukin- 10
Interleukin- 10 (IL-10) is produced by Th-1 and Th-2, monocytes, macrophages and possesses a wide spectrum of highly expressed immunosuppressive effect. IL-10 reduces the bioactivities of Th-1 more than of Th-2. The anti-inflammative property of IL-10 is expressed in terms of its ability to lower the production of pro-inflammative cytokines, rise up the production of IL-1 antagonist receptors and decrease the adhesion of leucocytes onto endothelial cells activated by IL-1. IL-10 can stimulate the synthesis of IgE. IL-10 and IL-4 are synergists in terms of their inhibiting function in cellular immunity.
In various tumors it has been marked out that: the IL-10 level rises up; the T-killer activities, MHC antigen expressions, IL-12 production and IFN-g production drop down and the presentation process of the tumor associated antigens gets weakened. The high level of IL-10 production features a bad prognostic sign and generally corresponds with a rapid tumor growth.
IL-10 can render an inhibiting effect in the growth process of certain tumors. So, histological research of the regressed melanoma B 16, resulted after the introduction of IL-10, has revealed an absence of the CD4 + and CD8 + lymphocytes infiltration and a presence of the NK-cells infiltration in the tumour. These data to some extent can explain various results of IL-10 in tumoral process.
IL-10 has an opposite effect on malignant and non malignant cells. IL-10 on one hand prevents apoptosis in B - cells of embryonic centres of lymphonodules through a bcl-2 protein induction, but on the other hand it induces apoptosis and inhibition of proliferation in B- cells during CLL.
Interleukin- 11
Interleukin- 11 (IL-11) is a pro-inflammative factor which regulates the functions of T- and B- lymphocytes, takes part in the induction of various killer cells' activities, acts as an autocrine factor for the proliferation of megacaryocytes. It takes part in an induction of acute phase protein synthesis just like IL-1 and IL-6. The role of IL-11 in the tumor growth is not well investigated yet.
Interleukin-12
Interleukin-12 (IL-12)- a polypotent activator of the cellular immunity with antitumoral and antimetastatic activities. It strengthens the bio-activity of T-killers, NK- and LAK-cells. IL-12 also activates the cytotoxicity of macrophages, where as the deficiency of its production by macrophages can considerably reduce the antitumor activities. IL-12 renders antitumoral effect in lung cancers. Intensification of the tumour growth, particularly in rectum cancer, associates with drop in the production of IL-12 and increase in IL-10 production. The important property of IL-12 is the intensification of FasL expression and induction of apoptosis. The recombinant IL-12 is capable to check the metastases into lungs and lymphatic nodules. The highest antitumor effect of IL-12 is observed during its combined action together with IL-2 and IFN-g.
IL-12 inhibits angiogenesis. Angiogenesis with the help of IL-12 takes place on the level of protienkinesis receptors, adhesive molecules, integrins and other surface structures, which increase the IFN-g production.
IL-12 suppresses the development of cachexy and anemia induced by IL-1b and IL-6 in the cancer patients.
Interleukin-13
Interleukin-13 (IL-13) is very sensitive to the monocytes and B-lymphocytes. It induces the phosphorylation, possesses many biological effects similar to IL-4 and the IL-13 receptor can be a sub unit of IL-4 receptor. The affinity of IL-4 and IL-13 to connect with the identical cells depends on the number of isoforms of receptor with which these interleukins connect. IL-13 does not act on T- lymphocytes. IL-13 inhibits the proliferation of leukemic pro-B-cells.
Interleukin-14
Interleukin -14 (IL-14) is a B-cell growth factor (BCGF). The hyper production of this interleukin enables the progression of B-cell type non Hodgkin's lymphoma (NHL-B). Where as, the antibodies to IL-14 slows down the growth of NHL-B.
Interleukin-15
Interleukin-15 (IL-15), in the biological properties, is very much analogous to IL-2 and in many respects they act as synergists- particularly in LAK-cells induction process. IL-15 increases the antitumor activities of T-killers and NK-cells, production of the cytokines CD4 + lymphocytes and can manifest to be a chemoattractant for T-lymphocytes. The production of endogenous IL-15 is one of the key conditions for a IFN-g synthesis. The receptors of the MHC class 1-type are expressed on the cytotoxic T-lymphocytes and NK-cells, which inhibit their biocidal activity – killing inhibiting receptors (KIR). IL-15 is capable to influence the expression of KIR.
Interleukin-16
Interleukin-16 (IL-16) is a T-cell chemoattractant. The main producers of IL-16 are the monocytes, CD8 + and B-lymphocytes, B- lymphocytes. This interleukin increases the mobility of CD4 + lymphocytes and together with IL-2 promotes their activation. A high level of IL-16 in blood serum is found in the patients with III and IV stages cancers of: intestine, kidney, urinary bladder, uterus, ovary and the breasts. Interferon alpha (IFN-a), histamine and serotonin increase the production of IL-16.
Interleukin-17
Interleukin-17 (IL-17) is principally produced by CD4 (+) T- cells which induces granulopoiesis via granulocyte colony stimulating factor (G-CSF). IL-17 takes part in the regulation of many cytokines - IL-1, IL-4, IL-6, IL-10, IL-12, IFN-g. IL-17 can reinforce the antibody dependant tumor cell destructions. Histamine and serotonin increase the production of IL-17.
Interleukin-18
Interleukin-18 (IL-18) acts as a synergist with IL-12 in some of their effects, especially in the induction of IFN-g production and inhibition of angiogenesis. A high IFN-g production under an integrated effect of IL-18 and IL-12 suppresses the tumor growth
Interleukin-19
Interleukin-19 (IL-19) is produced mainly by monocytes and in its biological function is similar to IL-10. The lipopolysaccharides (LPS) stimulate the synthesis of this interleukin. The strongest strong stimulator of IL-19 is GM-CSF. IL-19 regulates the functions of macrophages, suppresses the activities of Th-1 and Th-2.
IL-19 increases the synthesis of bcl-2 protein and thus influences in apoptosis of both tumoral and the immune cells.
Interleukin-20
Interleukin-20 (IL-20) is mainly secreted by the keratinocytes and plays an important role in skin inflammations. For example, IL-20 synthesis increases in psoriasis. In its bioactivities IL-20 is similar to IL-10 and can stimulate the tumor growths.
Interleukin-21
Interleukin-21 (IL-21) executes an important role in the regulation of haematopoiesis and in an immune response, influences in a development of lymphocytes. In terms of the bio-activities in antitumoral defence system, it is closest to IL-2 and IL-15
IL-21 promotes a high production of T-lymphocytes, promotes a fast growth and maturation of NK-cells as well as a fast growth of mature B-lymphocyte population.
Interleukin-22
Interleukin-22 (IL-22) is produced by activated T-lymphocytes in an acute stage of inflammation. In its bioactivities, it is similar to IL-10 to some extent, but unlike IL-10, IL-22 does not prohibit the production of pro inflammatory cytokines through monocytes in reply to LPS. Besides, in its bioactivities IL-22 is, to some extent, similar to interferons α, β and γ. The role of IL-22 in the antitumoral protection is not yet established.
The impact of interleukin in tumor growth is rather multifarious, therefore the immunotherapy of tumors with the help of interleukins should be approached with a definite knowledge of the initial level of the interleukin and its complex interaction with particular type of tumors and the immune cells.
It is important to know, that the use of high doses of interleukin causes side effects such as: algor, nausea and vomiting hyperbilirubinemia, oliguria, increase of kreatinine level, disorientation and low pressure. The formation of antibodies against the recombinant interleukins considerably reduces efficiency of their application.
Apoptosis
In an organism of a healthy person the cellular homeostasis is defined by a balance between the destructive and proliferative processes of cells. Apoptosis is a life long programmed, energetically dependent and genetically regulated cell destruction process. This cellular destruction process works through a special signal and gets rid of weak, unnecessary and damaged cells from an organism. Every day, approximately 5 % cells of an organism under go apoptosis, and their place occupy new cells. During this process the cells die away within 15-120 minutes without leaving any marks behind. TNF-a and Fas-ligand (CD178) switch on a cascade of biochemical reactions, whose final stage includes a chromosome defragmentation and consequently a complete loss of the cell. On the surface of cells present special receptors for TNF-a: TNF-RI (molecular weight- 55-60 kDa) and TNF-RII (molecular weight- 75-80 kDa), where as for a Fas-ligand exists a receptor so called Fas/APO-1 (CD95).
TNF-R and Fas/APO-1 (CD95) have homology in extracellular domains, represented in a cysteine form, rich in domains and homologous sequence of the intracellular parts of the receptor.

Fig. 7. Apoptosis
The connection of TNF-a and Fas-ligands with the apoptosis receptors activates the intracellular components of these receptors so called the "death effector domain" - DED: DED, DED1 and DED2 and series of intermediators, switching on the ceramides, ras, SAPK/JNK, protein tyrosine kinases, cathepsin D and proteases of the ICE/CED-3 family, which in turn pass down a death signal through a cascade system. The cysteic proteases of ICE/CED-3 family are located in the intracellular part of the apoptosis receptor in an inactive form. They are called the interleukin-lb converting enzyme (ICE). This ICE/CED-3 family include different types of proteases, many of them have more than one denotations. The cysteine-asparat protease family is also known as Caspase.
Altogether 14 different Caspases are known:
Caspase-1 (ICE)
Caspase-2 (Ich-1, Nedd2)
Caspase-3 (CPP32, Yama, Apopain, SCA-1, LICE)
Caspase-4 (ICEreI-II, TX, ICH-2)
Caspase-5 (ICErel-III, TY)
Caspase-6 (Mch-2)
Caspase-7 (Mch-3, ICE-LAP-3, CMH-1)
Caspase-8 (FLICE, Mach-1, Mch5)
Caspase-9 (ICE-LAP6, Mch6, Apaf-3)
Caspase-10 (FLICE-2, Mch4)
Caspase-11
Caspase-12
Caspase-13 (ERICE)
Caspase-14 (Mini-ICE)
Beside the Caspase family, Bcl-2 protein family also take part in the regulation of the apoptosis, where Bcl-2, Bcl-xL, Ced-9, Bcl-w and Mcl-1 proteins inhibit the apoptosis while the Bcl-2 homology (BH) 1-3, Bax like protein, Bak, Bok and consisting only the BH3 regions, Bad like protein, Bid, Bik, Bim and Hrk perform pro apoptosis functions. The activation of DED, DED1 and DED2 triggers a cascade modification and activation of proteases ICE/CED-3 family. The first step is the conversion of the non active pro-caspase-8 into the active caspase-8. The caspase-8 then activates caspase-3 and Bid. Bid further interacting with Bax enables the exit of cytochrome C from mitochondria, which activates caspase-9. The activated Caspase-9 in turn helps to release active caspase-3, -6, -7. In turn, the active ICE start to interact with a series of intracellular substrates: poly- (ADF-ribose) polymerase (PARP), participating in a DNA reparation and modification of activities of some of the nuclear proteins, lamin B1, topoisomerase I and P-actin. All the members of ICE/CED-3 protease family contain the catalytic ends of cysteine and split up the substrates next to the aspartic acid end in P1 position. The specific decomposition of PARP, lamina B1, topoisomerases I and P-actin under the action of ICE-like proteases on the large and small fragments cause a cell death, as the large fragments of these substrates also act as the active nucleases which split up chromosomes into fragments. For example, PARP split up CPP32/Yama into two fragments 85 and 24 Da, among which the apoptosis-specific one is the fragment with 85 kDa.
The activation of proteases ICE/CED-3 family can take place under the effect of phospholipids, for example, the ceramides which are capable to activate CPP32/Yama.

Fig. 8. Ceramides
The free sphingosine derived from ceramides as a result of hydrolysis by ceramidase can also activate ICE-like proteases and accelerate the apoptosis.


Fig. 9. SphingosineThyroxine (T4) plays an important role in an efficient performance of the apoptosis.
Fig. 10. Thyroxine
It regulates the functions of the protein thyrosinekinase, an important element for implementation of death signals. The apoptosis is suppressed at a deficiency of this thyroid hormone.
IL-lb prevents the apoptosis. The ICE-like proteases interact with IL-lb, instead of with PARP, lamina B1, topoisomerases I and P-actin. Because of this no active nucleases are formed and the cell avoids apoptosis.
The interaction of TNF-a and Fas-ligands with TNF-R and Fas/APO-1 (CD95) as well as the conduction of apoptosis signals take place under the influence of Bcl and Bax proteins. So the proteins of Bcl family: Bcl-2, Bcl-xL and Bcl-xS block off the cytochrome C exit from mitochondria which in turn checks the pro-capase-9 turning into active form, and revoke the apoptosis signal.
On the other hand the Bax proteins promote the cytochrome C exit from the mitochondria and formation of active caspase-9 which supports and further activates the apoptosis cascade, which has been initiated after connecting TNF-a or Fas-ligands with TNF-R and Fas/APO-1 (CD95). Whether the apoptosis takes place or not depends in the proportion of Bcl and Bax proteins in mitochondria. The predominance of Bcl protein expression blocks out the apoptosis outset where as the predominance of Bax protein expression promotes the implementation of death signals.
All these features are necessary to take into account during the antitumoral therapy.The understanding of the mechanism of apoptosis helps to choose out the right elements for a complex immunotherapies of cancers or to develop new means of treatment of this deadly disease. For example, the application of TNF-a will be inefficient for those patients who have high level of Bcl-2 and - or IL-lb. The R&D directed to increase the expression of Bax protein and reduction of Bcl-2 and/or IL-lb level would raise up the efficiency of TNF-a therapy.
Telomerase
One of the fundaments of life is the cell division in organism. All the information of an organism are stored in the chromosomes, whose basic elements are the deoxyribonucleic acids (DNA). The high polymerous DNA in a complex of numerous protein molecules constitutes a chromosome.
In 1932 Hermann Myollar, the nobel prize winner Genetic, paid attention on specific nature of the end parts of chromosomes which precluded the adhesion of one chromosome with another. He named them as "Telomere", that in Greek means " end parts ".
Before a cell division takes place, it is necessary for the chromosomes to duplicate with a help of an enzyme so called DNA-polymerase. All known DNA-polymerases carry out synthesis of DNA in the direction from 5' to 3' end and for this they require single chain DNA- matrix and a 3'-OH end of a primer, initial point for attachment of enzyme to nucleic chain. The function of a primer is performed by RNA, formed by an enzyme replicative complex – primase. After the synthesis of DNA duplicates the RNA- primer gets detached, and thus the newly formed daughter DNA chains happen to be not fully replicated, i.e. they become shorter than the DNA mother chain by a size of one RNA- primer (100-200 nucleotides) which determines the aging process of an organism.
The progressive truncation of the telomere limits the number of mitotic divisions and thus plays a role of a stopwatch which counts down the number of cell divisions and the life span of an organism. In each cell divisions the telomeres of the daughter cells become shorter by 100-200 nucleotides.
After reaching a critical length of the DNA telomere, a process is triggered which stops the further cellular divisions. It was first established in 1965 by an American scientist L. Heflik from the Institute of Vistar, Philadelphia. The human fibroblasts and epithelial cells, in a cultures in vitro, after 50-60 divisions (so-called "the Heflik Number") is permanently halted in G1- or G2-phases of the cell division. This permanent cell growth arrest is known as the senescence (end replication problem). It is associated with a loss of the telomere and formation of the "sticking" ends of the chromosomes which causes end-to-end fusion and generates the genomic instability, in turn the cell loses its reproductive function and dies away.
In an organism there are cells which can divide forever with out being liable to the aging process. They are the hematopoietic stem cells, activated lymphocytes, basal cells of a epidermis, male and female reproductive cells. The initial length of the telomeres are re-established in these cells with a help of the enzyme- telomerase. Such cells are peculiar in their ability to under go endless cell divisions. In other words, the elongation ( re-establishment) of the repeating telomere fragments (TTAGGG nucleotides) of DNA with an active telomerase elides the limitations in the number of cell divisions and such cells become immortal. This phenomenon is called immortality.
The limitations in the number of cell divisions is revoked in the malignant neoplasm as well, due to the activation of the telomerase genes, and thus such cells are also accessed to immortality. The active telomerase in alliance with suppressed apoptosis in cancer cells results into a tragical consequence for an organism, i.e. it dies away from an uncontrollable growth of the malignant tumor cells.
The enzyme - telomerase consisting of a protein part and RNA was discovered by Grader and Blackber in 1985. In a human being the hTERT (Homo sapiens telomerase reverse transcriptase) is constructed as an unreplicated 3'- end telomere of a DNA with short fragments of TTAGGG successions. The main function of the telomere is to protect the distal parts of the chromosomes from a degradation and sticking up during the cell division. The length of the telomere differs from 5 to 15 thousand pairs of bases (-OH). Namely the 3'- OH ends of the maternal chromosomes are identified by the telomerase which build up the maternal chain in hundreds of reiteratives, utilizing these 3'-OH ends as primers and RNA, a component of enzyme, as a matrix. In this way formed, the lengthy single-chained ends, in turn, serve as matrices for the synthesis of daughter chains according to the well known replicating mechanism.

Fig. 11. Re-establishment of telomerewith the help of telomerase
Today many research works to inhibit telomerase activities of the tumor cells are being carried out. By solving this hard but well accessible problem will enable to prolong considerably the life span of oncological patients.
The human intellectuals on one hand, have created thousand substances which may cause cancers, and on the other hand, have discovered many mechanisms of cancer growth. Such deep knowledge of interaction between the immune system and the cancers allows us, today, to choose an optimum methods of cancer treatment and essentially prolong or even save the lives of many of these hapless patients.